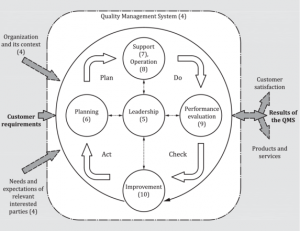
ISO 9001:2015 Quality Management System (QMS)
The implementation of a Quality Management System such as that accredited to the ISO 9001:2015 standard is the key to the successful management of quality performance within any organisation.
ISO 9001:2015 provides an established framework that can be developed to provide a full Quality Management System that can be used to monitor and improve working practices. It is a management tool that should be implemented through all levels of the organisation to help achieve the objectives of the organisation.

Achieving ISO 9001 can help to demonstrate that a company has the management policies and associated procedures in place to enable them to consistently achieve the standards set out by their clients.
ISO 9001 has been designed to be compatible with other management system standards and specifications, such as ISO 45001:2018 Occupational Health & Safety and ISO 14001 Environmental. There is a significant amount of commonality between the standards which lends itself to the development of one Integrated Management System.

At Your Safety Advisor Ltd, we have an experienced ISO Qualified Lead Auditor who is responsible for the development of all of our ISO Management systems
Why do you need an ISO 9001:2015 Quality Management System or a Health and Safety Management System such as ISO 45001?
Well, very simply because for most companies having an ISO 9001:2015 Quality Management System makes good business sense! Having an ISO registered Management System immediately demonstrates to your potential customers and suppliers that you are a professional organisation with robust and tested Management System in place. It also goes to show that you have audit trails and checks in place throughout your company procedures to help manage and control quality.
So, if you
- are a growing business?
- need to restructure?
- need to achieve ISO accreditation or CE certification?
- or simply need to comply with a client request to demonstrate the accreditation of your management systems,
Then ISO 9001:2015 and/or ISO 45001 will help you achieve these goals.
ISO 9001:2015 specifies the requirements for your Quality Management System (QMS) while ISO 45001:2018 specifies the requirements for your Occupational Health and Safety (OHS) Management System.
ISO Certified systems such as ISO 9001:2015 and/or ISO 45001 specify the requirements for a quality management system when an organization:
a) needs to demonstrate its ability to consistently provide products and services that meet customer and applicable statutory and regulatory requirements, and
b) aims to enhance customer satisfaction through the effective application of the system, including processes for improvement of the system and the assurance of conformity to customer and applicable statutory and regulatory requirements.’
Our Consultants and Quality Management Auditors offer a personalised business support service, providing you with a complete package from start to finish, including the final ISO accreditation. We can also provide ongoing support and monitoring to help ensure that your systems grow as your company expands. We can also help to ensure that the systems are properly maintained and implemented through out the company to ensure that your ISO Accreditation is retained during subsequent audits.
Improve your business reputation and meet the commonly accepted quality management criteria when tendering for work. ISO 9001:2015 provides you with an internationally recognised framework to monitor and improve working practices.
- ISO 9001:2015 and ISO 45001:2018 Accreditation are a useful and powerful marketing tool.
- An accredited Quality Management System (QMS) will often allow you to be listed as an Approved Supplier.
- It opens up access to new markets.
If agreed in advance with your Certification Body, ISO 45001:2018 Accreditation can even meet the requirements of the SSIP Accreditation and allow you to gain CHAS, SafeContractor, SMAS, Exor etc under the Deem to Satisfy Arrangements.
The main content of your QMS can generally be contained within the following sections:
- Document control
- Ordering Process
- Training
- Customer Focus
- Management Commitment
- Process Management
- Records
- Data
- Analysis and Monitoring
- Non-conformance’s
- Corrective & preventive actions
The basic steps involved in developing, implementing and then certifying your Quality Management System can be summarised as follows:
- Initial Audit and Gap analysis (Identify your core business processes)
- Development of your management system
- Implementation of the new management system
- Provision of staff training to help ensure that the procedures are being performed as they are described in your QMS
- Verify that your system is effective (Auditing of your new systems)
- Register your system and obtain certification from a third party accreditation provider